Penicillin Allergy Risk Low? Challenge with PO!

More evidence supports that proceeding directly to an oral penicillin challenge in patients who are low risk for penicillin allergy. This review highlights two studies using allergy history-taking to determine eligibility for direct oral penicillin challenge.
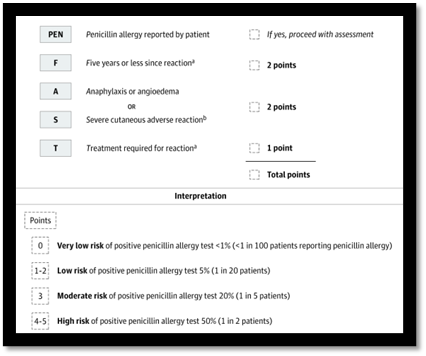
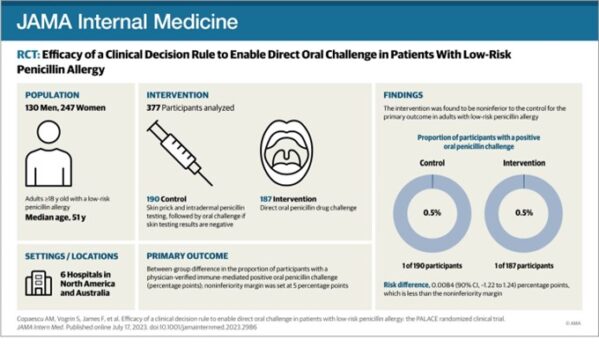
In the PALACE randomized clinical trial, investigators selected participants reporting low-risk penicillin allergies via PEN-FAST tool in this multicenter, non-inferiority, randomized clinical trial to undergo direct oral challenge (intervention) or skin-testing followed by oral challenge if the skin test is negative (control; standard-of-care). PEN-FAST is a short questionnaire validated to assess risk for penicillin allergy in patients reporting such an allergy; those deemed “low” and “very low” risk of a positive allergy test were eligible. A total of 382 outpatient adults across 6 medical centers in Australia, US and Canada were randomized. One patient in each group (0.5%) had a positive oral penicillin challenge (primary outcome); both were treated successfully with oral antihistamines. The trial met their non-inferiority margin of 5%. There was no difference in delayed immune reactions up to 5 days. These findings offer clinicians without the time or resources to implement penicillin skin testing as a safe alternative to skin-testing, which may be easily adaptable to inpatient settings.
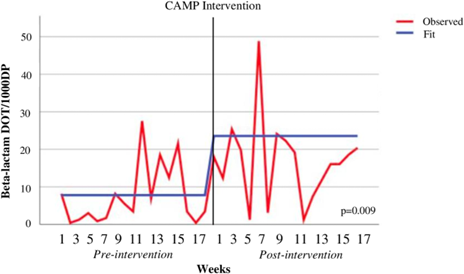
In an observational, quasi-experimental study at a non-teaching community medical center in New Jersey, investigators created a medication allergy history interview questionnaire and a medication allergy assessment algorithm to classify patients as low vs moderate vs high risk of allergy. Low risk patients could undergo oral beta-lactam challenge, moderate risk patients were referred for skin testing, and high-risk patients continued avoidance. The objective of this intervention was to increase allergy history documentation in the electronic medical record and increase beta-lactam use. One-hundred eighty-four patients in the pre-intervention period was December 2018 to April 2019 were compared to 208 in the January-April 2021 post-intervention period. Using interrupted time-series analyses, they found that documentation of complete allergy histories increased by nearly 20%, and beta-lactam use increased by 9.3 days of therapy per 1000 days-present. This study emphasized that non-teaching, community hospitals can successfully implement beta-lactam allergy interventions and inspired greater confidence among their clinicians in proceeding to direct oral challenge in low-risk individuals.
Reference:
Copaescu AM, Vogrin S, James F, et al. Efficacy of a Clinical Decision Rule to Enable Direct Oral Challenge in Patients With Low-Risk Penicillin Allergy: The PALACE Randomized Clinical Trial. JAMA Intern Med. 2023;183(9):944–952. doi:10.1001/jamainternmed.2023.2986
Trubiano JA, Vogrin S, Chua KYL, et al. Development and Validation of a Penicillin Allergy Clinical Decision Rule. JAMA Intern Med. 2020;180(5):745–752. doi:10.1001/jamainternmed.2020.0403
Vyas, L., Raja, K., Morrison, S., Beggs, D., Attalla, M., Patel, M., & Philips, M. (2023). Beta-lactam comprehensive allergy management program in a community medical center. Antimicrobial Stewardship & Healthcare Epidemiology, 3(1), E189. doi:10.1017/ash.2023.461
Article Submitted by Jenna Preusker, PharmD, Nebraska ASAP




