Infectious Diseases Society of America Community – Acquired Pneumonia Clinical Pathway Overview

In 2019, the Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) released a clinical practice guideline for diagnosis and treatment of community-acquired pneumonia (CAP). Many notable updates were made, including the elimination of the term healthcare-associated pneumonia (HCAP) that was introduced in 2005. Instead, emphasis was placed on evaluating individual and local risk factors for methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa. Another update was made regarding procalcitonin, with the guideline recommending initiation of therapy in radiographically confirmed and clinical suspected pneumonia regardless of procalcitonin.
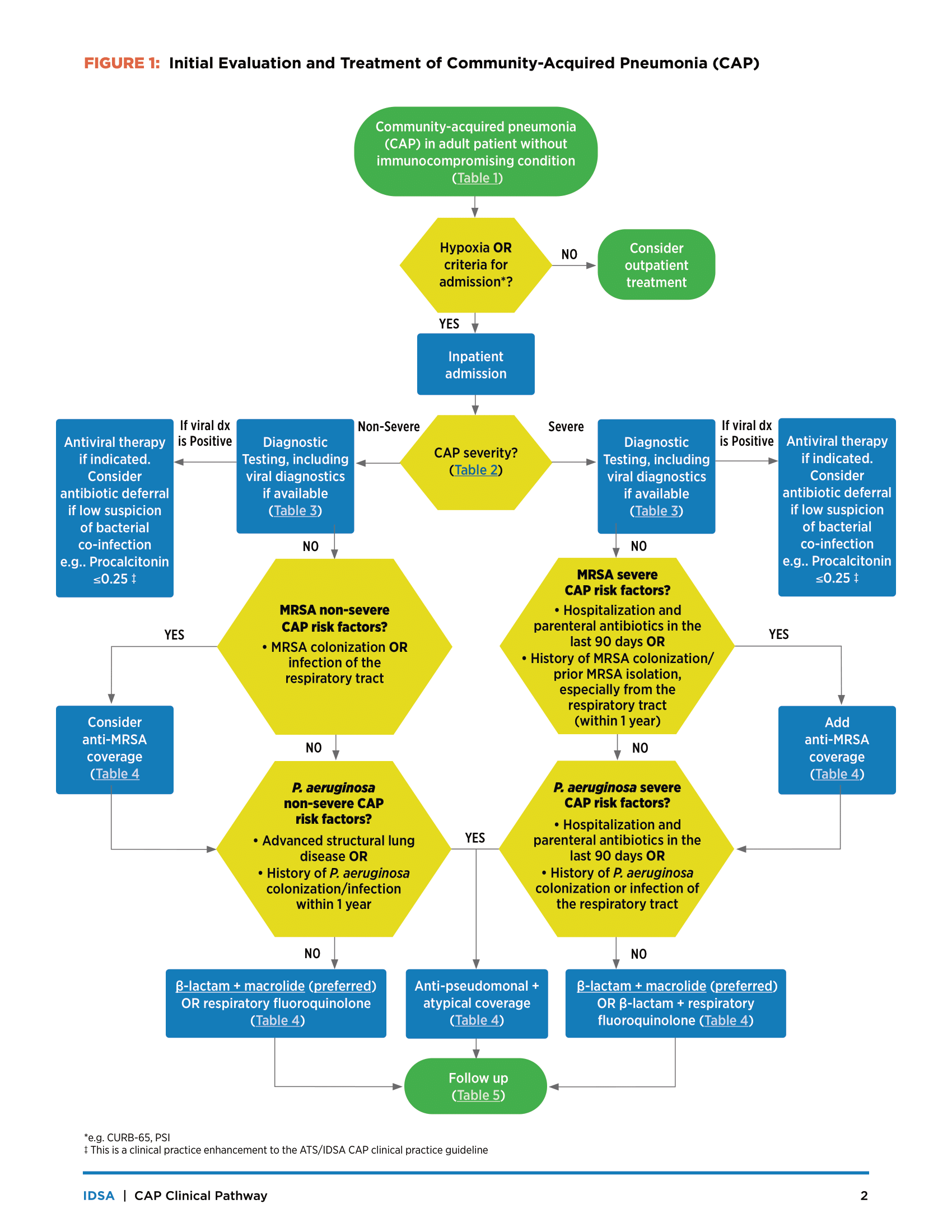
In October 2023, the IDSA released a CAP clinical pathway tool to help facilitate the implementation of CAP guidelines in hospitalized patients. This tool was created with funding from the CDC by a group of 12 volunteers with clinical expertise in antimicrobial stewardship and infectious diseases. Feedback on feasibility and usability was obtained and it was piloted through 9 facilities. The clinical pathway is consistent with the 2019 CAP guidelines, with some enhancements. It can be used to help guide therapy options for CAP and reduce variability in prescribing. Notably, immunosuppressed patients are excluded from this clinical pathway, a definition of immunosuppressed patients is provided.
The CAP clinical pathway tool starts with diagnosis of CAP, which is defined as a newly recognized pulmonary infiltrate(s) on chest imaging plus at least one respiratory symptom plus at least one other symptom/sign or finding defined in Table 1 of the pathway. It then moves on to assessment of CAP severity, essentially boiling down to if the patient requires ICU level care or not (criteria can be found in Table 2). Next, diagnostic testing is discussed and another table is provided (Table 3), which succinctly details what diagnostic tests should be considered for both non-severe and severe CAP. This section also includes some clinical practice enhancements to the 2019 guideline. These enhancements include when to obtain procalcitonin, molecular testing for bacterial pathogens, COVID19 testing, and expanded viral molecular panel testing.
Notably, this enhancement recommends consideration of antibiotic deferral in patients with positive viral testing, a low suspicion of bacterial infection (e.g., procalcitonin < 0.25 or an 80% reduction on repeat testing in 72 hours), and WBC < 10,000 cells/µL.
Next, the pathway moves on to CAP treatment, and further defines when empiric therapy for MRSA and P. aeruginosa should be initiated. Initiation of empiric coverage for these organisms are stratified by CAP severity and are separately addressed for each organism, emphasizing that empiric therapy should not automatically be started for both organisms if risk factors for only one of the two organisms are present. Table 4 outlines treatment options for CAP, which remain largely unchanged from the 2019 guidelines. Emphasis is placed here on preference for beta-lactam + macrolide therapy including recommendations for close evaluation of beta-lactam allergies at the top of the table. In addition, see our blog post from Tuesday this week for further information on evaluation of penicillin allergies. The pathway addresses duration of therapy for azithromycin, recommending 500 mg daily for 3 days if Legionella is not identified or an alternate etiology is identified.
Lastly, the clinical pathway enhances the guidelines by including one last part in the pathway: Daily follow-up stewardship considerations. These recommendations can be found in Table 5 and include considerations for each step in the pathway. Assessing for clinical improvement, determining pathogen specific therapy based on diagnostics and cultures, considerations for when to transition to oral antibiotics, and consideration for vaccines are included, among many others. Oral step-down antibiotics are listed, including choices for no MDRO risk factors and MDRO risk factors, and recommended doses. Lastly, duration of therapy is addressed and has been enhanced from the 2019 guidelines. The 2019 guidelines recommended 5-7 days of therapy; however, the new clinical pathway recommends 3-5 total days of therapy if the patient is clinically stable by day 3.
Ways to Use the IDSA CAP Clinical Pathway
- Make the pathway into a pocket card
- Use the pathway to make order sets in an EHR
- Use application-based implementation
- Utilize it for education emphasizing 2-3 main points (e.g. allergy assessment and duration of therapy). Use to help drive quality improvement initiatives (reducing duration of therapy, earlier oral step down, etc.)
The clinical pathway tool including all the tables mentioned above is available here.
Submitted by Danny Schroeder, PharmD, BCPS
Nebraska ASAP Antimicrobial Stewardship Pharmacist