Syphilis

- STIs including syphilis are increasing across the US, including Nebraska!
- Use our ASAP resources to:
- Review appropriate syphilis treatment
- Manage the nationwide injectable penicillin shortage
- Better manage a patient with a history of reported penicillin allergy
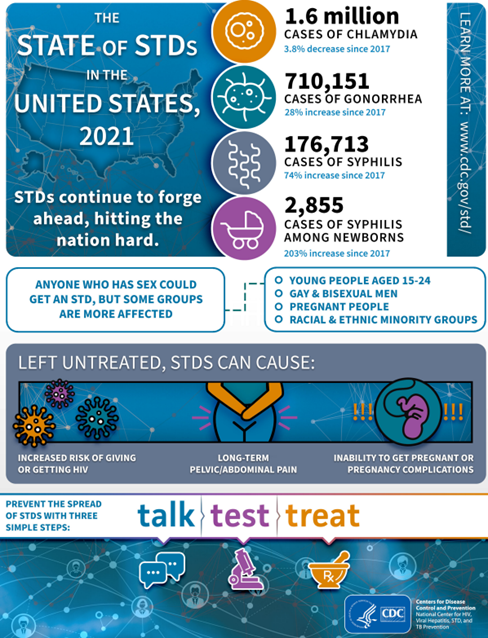
Image source: TheStateOfSTDs.psd (cdc.gov)
Section I: Syphilis Treatment
Treatment
Penicillin G, administered parenterally, is the preferred drug for treating patients in all stages of syphilis. The preparation used (i.e., benzathine, aqueous procaine, or aqueous crystalline), dosage, and length of treatment depend on the stage and clinical manifestations of the disease. Treatment for late latent syphilis (>1 years’ duration) and tertiary syphilis requires a longer duration of therapy. Longer treatment duration is required for persons with latent syphilis of unknown duration to ensure that those who did not acquire syphilis within the preceding year are adequately treated.
Primary, Secondary, or Early Latent (<1 year)
Benzathine penicillin G 2.4 million units IM in a single dose
Late Latent (>1 year), Latent Syphilis of Unknown Duration, or Tertiary Syphilis with Normal CSF Examination
Benzathine penicillin G 7.2 million units total, administered as 3 doses of 2.4 million units IM each at 1-week intervals
Neurosyphilis, Ocular Syphilis
Aqueous crystalline penicillin G 18-24 million units per day, administered as 3-4 million units IV every 4 hours or continuous infusion, for 10-14 days
- Additional doses of benzathine penicillin are not indicated in patients with HIV infection
- Additional doses of penicillin in pregnant women with early syphilis may be indicated if evidence of fetal syphilis on ultrasound
Selection of the appropriate penicillin preparation is important because T. pallidum can reside in sequestered sites (e.g., the CNS and aqueous humor) that are poorly accessed by certain forms of penicillin. Combinations of benzathine penicillin, procaine penicillin, and oral penicillin preparations are not considered appropriate for syphilis treatment. Reports have indicated that practitioners have inadvertently prescribed combination long- and short-acting benzathine-procaine penicillin (Bicillin C-R) instead of the standard benzathine penicillin product (Bicillin L-A) recommended in the United States for treating primary, secondary, and latent syphilis. Practitioners, pharmacists, and purchasing agents should be aware of the similar names of these two products to avoid using the incorrect combination therapy agent for treating syphilis.
Penicillin’s effectiveness for treating syphilis was well established through clinical experience even before the value of randomized controlled clinical trials was recognized. Therefore, approximately all recommendations for treating syphilis are based not only on clinical trials and observational studies, but on many decades of clinical experience.
Special Considerations
Pregnancy
Parenteral penicillin G is the only therapy with documented efficacy for syphilis during pregnancy. Pregnant women with syphilis at any stage who report penicillin allergy should be desensitized and treated with penicillin (see section on Managing Patients with a History of Penicillin Allergy).
Jarisch-Herxheimer Reaction
The Jarisch-Herxheimer reaction is an acute febrile reaction frequently accompanied by headache, myalgia, and fever that can occur within the first 24 hours after the initiation of any syphilis therapy; it is a reaction to treatment and not an allergic reaction to penicillin. Patients should be informed about this possible adverse reaction and how to manage it if it occurs. The Jarisch-Herxheimer reaction occurs most frequently among persons who have early syphilis, presumably because bacterial loads are higher during these stages. Antipyretics (such as acetaminophen) can be used to manage symptoms; however, they have not been proven to prevent this reaction. The Jarisch-Herxheimer reaction might induce early labor or cause fetal distress in pregnant women; however, this should not prevent or delay therapy.
Management of Sex Partners
Sexual transmission of T. pallidum is thought to occur only when mucocutaneous syphilitic lesions are present. Such manifestations are uncommon after the first year of infection. Persons exposed through sexual contact with a person who has primary, secondary, or early latent syphilis should be evaluated clinically and serologically and treated according to CDC recommendations Syphilis – STI Treatment Guidelines (cdc.gov)
Doxycycline Post-Exposure Prophylaxis

What is doxy-PEP?
Post-exposure prophylaxis (PEP) is a treatment administered after potential exposure to a disease to prevent it from developing. PEP differs from pre-exposure prophylaxis (PrEP), which involves taking medication before potential exposure to prevent infection.
Doxycycline is used as a first line treatment for chlamydia, a second line treatment for syphilis, and additionally has some efficacy against gonorrhea, making it an appealing option for use as PEP (known as doxy-PEP).
Which patients could be considered for doxy-PEP and how should it be prescribed?
- CDC recommends that providers should counsel gay, bisexual, and other men who have sex with men (MSM) and transgender women (TGW) with a history of at least one bacterial sextually transmitted infection (STI)(specifically syphilis, chlamydia, or gonorrhea) during the past 12 months about the benefits and harms of using doxy-PEP and use shared decision-making approach with MSM and TGW who have not had a bacterial STI diagnosed during the previous year but will be participating in sexual activities that are known to increase the likelihood of exposure to STIs.
- No recommendation can be given at this time on the use of doxy PEP for cisgender women, cisgender heterosexual men, transgender men, and other queer and nonbinary persons.
What should be covered at the initial postexposure prophylaxis visit?
- Pre-prescription screening: At initiation of doxy-PEP and every 3-6 months, screen for gonorrhea and chlamydia at all anatomic sites of exposure (urogenital, pharyngeal, and/or rectal), as well as testing for syphilis and HIV (if not known person living with HIV). If diagnosed with an STI, patients should be treated according to CDC STI Treatment Guidelines. For persons without HIV infection not receiving HIV PrEP, consider screening for HIV infection every 3-6 months.
- Provide comprehensive preventative sexual health counseling and education to all sexually active individuals including HIV/STI screening, doxy-PEP
- HIV pre-exposure prophylaxis CDC HIV PrEP Guidelines
- HIV post-exposure prophylaxis CDC PEP Guidelines
- Vaccinations (e.g. Hepatitis A/B, Human Papilloma Virus (HPV), Meningococcal, Mpox)
- Expedited partner therapy (Legal Status of EPT – Nebraska (cdc.gov))
- Prescribe 200 mg of doxycycline to be taken ideally within 24 hours (no later than 72 hours) after oral, vaginal, or anal sex. Doxycycline can be taken daily depending on sexual activity, but no more than 200 mg every 24 hours.
- Any formulation can be used: Doxycycline hyclate or monohydrate immediate release 100 mg (2 tabs taken simultaneously)
- Immediate release is typically less expensive than delayed release. Doxycycline hyclate delayed release 200 mg (1 tab) while more expensive may have fewer gastrointestinal side effects.
- Provide enough doses of doxycycline to last until the next follow-up visit, based on individual behavioral assessment through shared-decision making
How should patients receiving doxy-PEP prescriptions be counseled?
- People taking doxycycline should be counseled about possible drug interactions, risk of sun sensitivity, gastrointestinal symptoms, remaining upright for 30 minutes after taking doxycycline to reduce the risk of pill esophagitis, and the rare risk of benign intracranial hypertension and other serious side effects – doxycycline FDA label.
- Doxycycline is generally safe and well tolerated. Long-term use of doxycycline has been prescribed safely for many years for other medical indications such as acne treatment and malaria.
- All antibiotics carry a risk of causing Clostridioides difficile infection, but doxycycline has a much lower risk than other antibiotic classes.
- Counsel on the importance of separating the doxycycline dose by at least 2 hours from dairy products, antacids, and supplements that contain calcium, iron, magnesium, or bicarbonate.
- Doxycycline should not be taken during pregnancy due to the knowledge that tetracyclines cause cosmetic staining of the primary dentition in fetuses exposed during the second or third trimester of pregnancy and manufacturer concerns about possible enamel hypoplasia and depression of fetal bone growth. Doxy-PEP use should be avoided in women of childbearing age.
What should be covered at follow-up postexposure prophylaxis visits?
- Screen for gonorrhea and chlamydia at anatomic sites of exposure and syphilis every 3–6 months per CDC STI treatment guidelines recommendations for screening MSM and TGW.
- For persons without HIV receiving HIV PrEP, screen per CDC HIV PrEP guidelines (CDC HIV PrEP Guidelines). For persons without HIV infection not receiving HIV PrEP, consider screening for STIs and HIV infection every 3–6 months. Assess for the need for HIV PEP and encourage the use of HIV PrEP.
- Confirm or encourage linkage to HIV care for persons living with HIV infection.
- Assess for side effects from doxycycline.
- Provide risk reduction counseling and condoms.
- Re-assess continued need for doxy PEP.
Should we be worried about causing bacterial resistance by using doxy-PEP?
As this is a novel strategy, no data exists on the likelihood of creating antimicrobial resistance. Any use of antibiotics may lead to resistance. Data are being collected and reviewed for possible antimicrobial resistance among bacterial STIs (gonorrhea and chlamydia), commensal Neisseria (potential reservoir for tetracycline resistant plasmids), and community-acquired Staphylococcus aureus.
References and Additional Resources:
- Nebraska:
- CDC:
- California Prevention Training Center – Educational opportunities and training materials
Additional Resources
Section II: Penicillin Drug Shortage
Additional Resources
Section III: Managing Patients with a History of Penicillin Allergy
Treatment Guidelines
Penicillin is recommended for all clinical stages of syphilis, and no proven alternatives exist for treating neurosyphilis, congenital syphilis, or syphilis during pregnancy.
Penicillin allergy is one of the most frequently reported antibiotic allergies. It is often overreported, and the majority of patients who report penicillin allergy are able to tolerate the medication. Patients often are incorrectly labeled as allergic to penicillin and are therefore denied the benefit of a ß-lactam therapy. The presence of a penicillin allergy label considerably reduces prescribing options for affected patients. Moreover, penicillin allergy labels lead to the use of more expensive and less effective drugs and can result in adverse consequences, including longer length of hospital stay and increased risk for infection.
The overreported prevalence of penicillin allergy is secondary to imprecise use of the term “allergy” by families and clinicians and lack of clarity to differentiate between immunoglobulin E (IgE)-mediated hypersensitivity reactions, drug intolerances, and other idiosyncratic reactions that can occur days after exposure. Approximately 80% of patients with a true IgE-mediated allergic reaction to penicillin have lost the sensitivity after 10 years. Thus, patients with recent reactions are more likely to be allergic than patients with remote reactions, and patients who had allergic reactions in the distant past might no longer be reactive.
In a Baltimore, Maryland, STI clinic study, only 7.1% of the patients who reported allergy to penicillin or to another ß-lactam antibiotic had an objective positive test for penicillin allergy. Moreover, in studies that have incorporated penicillin skin testing and graded oral challenge among persons with reported penicillin allergy, the true rates of allergy are low, ranging from 1.5% to 6.1%.2-4 In hospitalized patients and other populations with comorbidities, the typical rates of validated penicillin allergy among patients who report a history of penicillin allergy are 2.5%–9.0%.6-8
Patients at Low Risk for Oral Challenge
If the patient gives only a low-risk history of IgE-mediated penicillin allergy that includes symptoms such as those listed below, an oral challenge (oral one-time dose of amoxicillin 250 mg) can be administered to document the absence of allergy. If the reaction occurred in the distant past (>10 years), the likelihood is reduced even further. The risk for severe amoxicillin-mediated anaphylaxis has decreased over time and is rare.
Low risk history in patients who report Penicillin allergy (Give Oral Challenge)
- Gastrointestinal Symptoms
- Headache
- Pruritis without rash
- Localized rash
- Delayed onset rash (>24 hours)
- Symptoms unknown
- Family history of penicillin or other drug allergy
- Patient denies allergy, but it is on the medical record
Similar to in-clinic vaccine administration, clinics planning to administer an oral amoxicillin challenge to low-risk patients should have a plan in place to manage potential reactions. Rescue medications including oral diphenhydramine and IM epinephrine should be readily accessible and clinic staff should be provided with education on reaction management steps. The clinic plan should include processes for transferring the patient to a higher level of care in the event of anaphylaxis (i.e. emergency department).
Reactions during oral amoxicillin challenge in low-risk patients are uncommon. In one study conducted in a primary care clinic, 3% of patients (3/99) receiving an oral amoxicillin challenge had minor cutaneous reactions (i.e. itching) and were treated with oral diphenhydramine (none received epinephrine).4
Monitoring the patient in the clinic for 60 minutes after ingestion of the oral amoxicillin challenge is recommended. Patients should be observed for life-threatening immediate reactions including hypotension and bronchospasm.9
Skin Testing for Penicillin Allergy
Skin testing for penicillin allergy should be performed if any indication exists that the symptoms were secondary to an IgE-mediated hypersensitivity. Testing is also indicated as a potential diagnostic procedure to definitively rule out penicillin allergy and document a negative allergy status in the medical record (i.e., delabeling). Penicillin skin testing has become a clinically significant element in antibiotic stewardship programs, and the procedure has been increasingly used by hospital-based pharmacists, hospitalists, and infectious disease physicians as part of overall antibiotic stewardship interventions. When integrated into stewardship, the rates of ß-lactam antibiotic use increased substantially.
Persons with negative results of a penicillin skin test, followed by an amoxicillin oral challenge, can receive conventional penicillin therapy safely if needed. Persons with positive skin test results and for whom no other clinical options exist (e.g., neurosyphilis and syphilis in a pregnant woman) should be referred to an allergist and desensitized before initiating treatment.
Desensitization
Desensitization is required for persons who have a documented penicillin allergy and for whom no therapeutic alternatives exist (e.g., syphilis during pregnancy and persons with neurosyphilis). Modified protocols might be considered on the basis of the clinical syndrome, drug of choice, and route of administration. Patients might require referral to a specialty center where desensitization can be performed.
References
- Gadde J, Spence M, Wheeler B, Adkinson NF Jr. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA 1993;270:2456–63
- Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract 2017;5:813–5
- Goldberg A, Confino-Cohen R. Skin testing and oral penicillin challenge in patients with a history of remote penicillin allergy. Ann Allergy Asthma Immunol 2008;100:37–43.
- Iammatteo M, Alvarez Arango S, Ferastraoaru D, et al. Safety and outcomes of oral graded challenges to amoxicillin without prior skin testing. J Allergy Clin Immunol Pract 2019;7:236–43.
- Trubiano JA, Thursky KA, Stewardson AJ, et al. Impact of an integrated antibiotic allergy testing program on antimicrobial stewardship: a multicenter Clin Infect Dis 2017;65:166–74.
- Siew LQC, Li PH, Watts TJ, et al. Identifying low-risk beta-lactam allergy patients in a UK tertiary centre. J Allergy Clin Immunol Pract 2019;7:2173–e1.
- Chen JR, Tarver SA, Alvarez KS, Tran T, Khan DA. A proactive approach to penicillin allergy testing in hospitalized patients. J Allergy Clin Immunol Pract 2017;5:686–93
- Leis JA, Palmay L, Ho G, et al. Point-of-care β-lactam allergy skin testing by antimicrobial stewardship programs: a pragmatic multicenter prospective evaluation. Clin Infect Dis 2017;65:1059–65.
- Gateman DP, Rumble JE, Protudjer JLP, Kim H. Amoxicillin oral provocation challenge in a primary care clinic: a descriptive analysis. CMAJ Open. 2021 Apr 16;9(2):E394-E399.



